Abdominal Aortic Aneurysm Repair Device Market Size, Production, Sales, Average Product Price, Market Share, Import vs Export

- Published 2025
- No of Pages: 120+
- 20% Customization available
Abdominal Aortic Aneurysm Repair Device Market: Overview of a Rapidly Evolving Sector
The abdominal aortic aneurysm repair device market is witnessing notable evolution, shaped by technological innovations and an expanding base of aging patients prone to vascular complications. As the occurrence of abdominal aortic aneurysms increases globally, the demand for safe, precise, and long-lasting intervention methods has intensified. This trend is placing the abdominal aortic aneurysm repair device market at the forefront of vascular treatment innovation, with companies focusing on enhancing device performance and reducing patient recovery times.
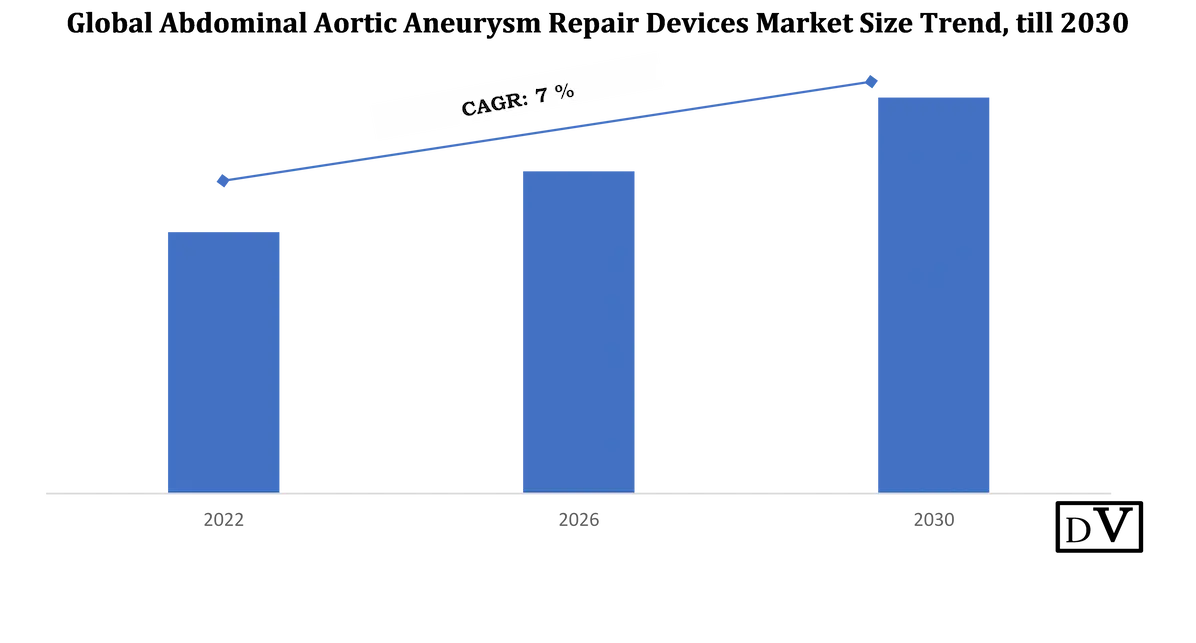
Expansion Driven by Minimally Invasive Procedures in the Abdominal Aortic Aneurysm Repair Device Market
Minimally invasive repair procedures are becoming the cornerstone of treatment in the abdominal aortic aneurysm repair device market. These procedures offer shorter recovery periods and reduced hospital stays, making them a preferred choice in clinical practice. This shift has led to a rising demand for modular endografts and catheter-based systems that align with these modern approaches. As clinical preferences move away from traditional open repair methods, the abdominal aortic aneurysm repair device market continues to adapt through specialized designs and flexible materials.
Product Development Reshaping the Abdominal Aortic Aneurysm Repair Device Market
Innovation remains central to the progression of the abdominal aortic aneurysm repair device market. With the need to treat increasingly complex patient anatomies, manufacturers are introducing advanced stent grafts with improved conformability and sealing mechanisms. Branched and fenestrated grafts have emerged as critical tools for physicians dealing with anatomically challenging cases. These advancements are positioning the abdominal aortic aneurysm repair device market as a key area of investment for medtech developers aiming to solve persistent clinical challenges.
Clinical Demand Steering the Abdominal Aortic Aneurysm Repair Device Market
Healthcare providers are actively prioritizing technologies that minimize procedure time while improving long-term outcomes. This emphasis has led to a notable rise in clinical trials and regulatory approvals targeting the abdominal aortic aneurysm repair device market. The market is seeing greater acceptance of customized devices tailored to individual vascular structures. As more physicians adopt these patient-specific solutions, the abdominal aortic aneurysm repair device market is expected to sustain its upward trajectory, especially in regions with high aging populations.
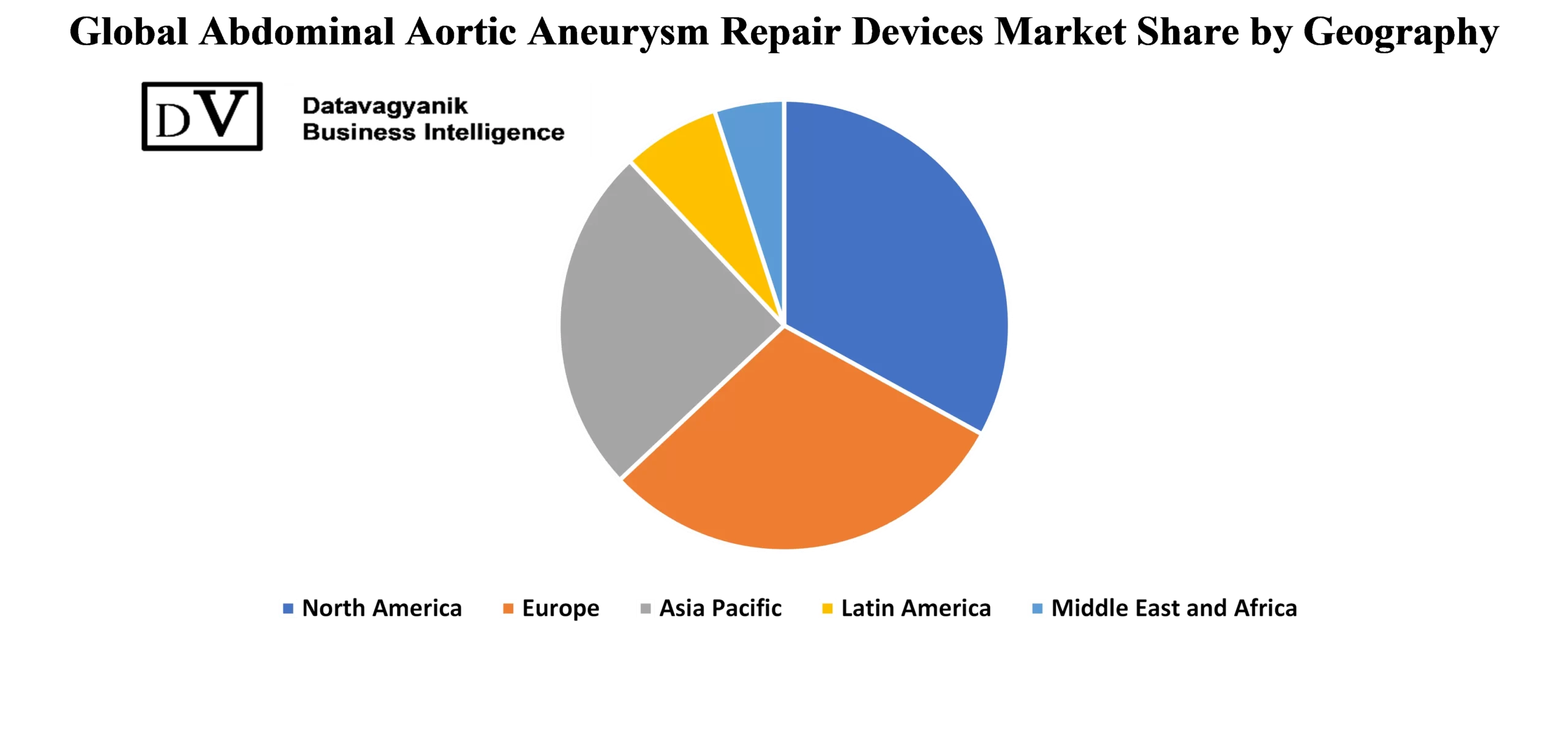
Regional Expansion Trends in the Abdominal Aortic Aneurysm Repair Device Market
Geographic expansion is a key growth lever within the abdominal aortic aneurysm repair device market. Developed economies continue to lead in device adoption due to advanced healthcare infrastructure and early diagnostic capabilities. However, emerging regions are now contributing to the abdominal aortic aneurysm repair device market through improved access to specialized care and increased awareness of vascular diseases. The expansion into these new territories is unlocking fresh revenue streams and pushing manufacturers to develop cost-effective devices for wider deployment.
“Track Country-wise Abdominal Aortic Aneurysm Repair Device Production and Demand through our Database”
-
-
- Abdominal Aortic Aneurysm Repair Device sales volume database for 30+ countries worldwide
-
Competitive Landscape in the Abdominal Aortic Aneurysm Repair Device Market
The abdominal aortic aneurysm repair device market is marked by a blend of established global players and rapidly emerging regional manufacturers. Larger firms are consolidating their market position through mergers, technology acquisitions, and global distribution networks. For example, multinational manufacturers are investing in next-generation devices with repositioning capabilities, advanced sealing mechanisms, and integrated navigation systems to meet the increasing demand for precision-guided repairs.
On the other hand, regional companies are competing by offering lower-cost alternatives without compromising on essential clinical outcomes. These firms are targeting healthcare systems under budgetary constraints, especially in Latin America, Eastern Europe, and Southeast Asia. This dual-competition model is intensifying across the abdominal aortic aneurysm repair device market, pushing all players to continuously innovate while maintaining competitive pricing strategies.
Product pipelines across leading players are increasingly focused on patient-specific devices, as anatomical variation remains one of the most significant clinical challenges. Companies are also enhancing their post-procedural monitoring tools, introducing smart grafts that facilitate imaging compatibility and post-implant surveillance. As the abdominal aortic aneurysm repair device market matures, innovation and operational agility will be critical differentiators.
Regulatory Landscape Shaping the Abdominal Aortic Aneurysm Repair Device Market
The abdominal aortic aneurysm repair device market is also influenced by evolving regulatory standards across regions. In high-income countries, regulatory pathways demand comprehensive clinical data and long-term outcome validation. This has resulted in longer product approval timelines, but also encourages the development of more reliable and safe repair devices.
Meanwhile, emerging markets are streamlining their approval processes to attract more global and regional players. Fast-track approvals and adaptive regulatory frameworks are creating new opportunities for device launches in markets such as India and Brazil. However, manufacturers must still demonstrate compliance with strict quality control protocols and device performance consistency.
Regulations are also impacting abdominal aortic aneurysm repair device manufacturing practices. Cleanroom certification, traceability protocols, and biocompatibility standards are now embedded in production processes. Manufacturers capable of maintaining these standards while reducing operational costs are gaining faster entry into multiple markets.
Future Trends Transforming the Abdominal Aortic Aneurysm Repair Device Market
The abdominal aortic aneurysm repair device market is transitioning into a more dynamic and patient-centric phase. Future trends include the integration of artificial intelligence in treatment planning, robotic assistance during endovascular procedures, and biodegradable graft materials. These innovations are expected to reduce procedure time, enhance precision, and lower long-term complication rates.
One notable shift is toward same-day discharge protocols. As healthcare systems aim to improve cost-efficiency, low-profile, rapid-deployment repair devices are gaining traction. These devices are designed for fast vascular access, limited surgical trauma, and shorter patient monitoring cycles. Their adoption is expected to increase by over 20 percent annually in outpatient centers.
In addition, wearable health technologies are beginning to integrate with vascular care. Smart monitoring systems that track post-repair blood flow, graft integrity, and vessel dilation in real time are under development. These systems, when combined with high-precision stent grafts, could redefine how the abdominal aortic aneurysm repair device market operates in the coming decade.
Investment Opportunities and Strategic Outlook in the Abdominal Aortic Aneurysm Repair Device Market
Investment momentum in the abdominal aortic aneurysm repair device market remains strong, particularly in companies developing platform technologies that allow for customization, integration, and scalability. Venture capital and private equity interest is rising in firms offering advanced 3D imaging-guided intervention tools and modular graft systems. These technologies not only address unmet clinical needs but also offer cost-effective manufacturing scalability.
Private-label partnerships are also on the rise, with larger manufacturers collaborating with regional hospitals to co-develop and trial new device configurations. These partnerships not only secure volume commitments but also provide real-world usage data that guide iterative product design.
There is a growing investment trend in upgrading abdominal aortic aneurysm repair device manufacturing facilities. Companies are enhancing their production capacity to accommodate rising demand while incorporating robotic precision, predictive analytics, and digital quality control systems. Manufacturing hubs in Southeast Asia and Eastern Europe are being equipped to serve both local and export markets.
Long-term growth in the abdominal aortic aneurysm repair device market is expected to be driven by three key forces: increasing global disease burden, advancements in medical imaging and navigation technologies, and a healthcare-wide shift toward value-based treatment outcomes. Manufacturers who align their innovation strategies with these macro forces are likely to lead the next wave of growth.
Conclusion: Strategic Positioning in the Abdominal Aortic Aneurysm Repair Device Market
The abdominal aortic aneurysm repair device market is entering a pivotal phase defined by customization, decentralization, and digital transformation. Regional demand variations, the expansion of minimally invasive repair techniques, and increasing investment in advanced materials are reshaping market fundamentals.
With procedural volumes increasing globally and life expectancy rising, demand for safe, efficient, and cost-effective repair solutions will only intensify. Companies that invest in flexible abdominal aortic aneurysm repair device manufacturing models, integrate feedback-driven design cycles, and maintain regulatory agility are likely to secure competitive advantages.
In an environment where innovation is both an opportunity and a necessity, success in the abdominal aortic aneurysm repair device market will depend on an organization’s ability to balance clinical performance with economic value. The coming years will test the agility, vision, and execution capabilities of every stakeholder involved in this highly specialized, yet globally critical, sector.
“Abdominal Aortic Aneurysm Repair Device Manufacturing Database”
-
-
- Abdominal Aortic Aneurysm Repair Device top manufacturers market share for 30+ manufacturers
- Top 10 manufacturers and top 20 manufacturers of Abdominal Aortic Aneurysm Repair Device in North America, Europe, Asia Pacific
- Abdominal Aortic Aneurysm Repair Device sales dashboard, Abdominal Aortic Aneurysm Repair Device sales data in excel format
-
Leading Players in the Abdominal Aortic Aneurysm Repair Device Market
The abdominal aortic aneurysm repair device market is highly competitive and moderately consolidated, with a handful of key manufacturers holding the majority of the global market share. These companies leverage advanced research capabilities, strong distribution networks, and long-term clinical data to maintain leadership positions. Most of the leading players offer a diverse portfolio of endovascular grafts and delivery systems, enabling them to cater to a wide range of patient anatomies and procedural requirements.
Companies dominating the global abdominal aortic aneurysm repair device market include Medtronic, Cook Medical, W. L. Gore & Associates, Terumo Corporation, Endologix LLC, and Becton, Dickinson and Company (through its subsidiary Bard Peripheral Vascular). These manufacturers have established brand recognition, regulatory approvals in multiple regions, and dedicated research arms focused on vascular innovation.
Manufacturer Market Share in the Abdominal Aortic Aneurysm Repair Device Market
Medtronic currently holds the largest share of the abdominal aortic aneurysm repair device market, accounting for approximately 28% of global revenue. The company’s key offerings, including the Endurant II and Endurant IIs stent graft systems, are widely adopted across North America and Europe due to their flexibility, conformability, and ease of use in various aortic anatomies. Medtronic has built its market dominance through consistent product enhancements and a global service infrastructure that supports device deployment and physician training.
Cook Medical follows closely with an estimated 22% market share. Its Zenith platform, including Zenith Flex, Zenith Alpha, and Zenith Fenestrated devices, has earned strong clinical adoption for complex and high-risk cases. Cook Medical has a reputation for offering highly customizable stent grafts, which are particularly valued in treating juxtarenal and thoracoabdominal aneurysms. Its extensive range and compatibility with patient-specific planning tools reinforce its competitive position.
L. Gore & Associates maintains around 18% of the abdominal aortic aneurysm repair device market, with its flagship product being the Excluder AAA Endoprosthesis. This device has gained traction for its low-profile delivery and durability in long-term performance. The company is also investing in devices suited for small and narrow-access vessels, allowing greater use in challenging cases or smaller patients.
Endologix LLC, though smaller in size, holds an important niche within the abdominal aortic aneurysm repair device market with its AFX Endovascular AAA System and ALTO Abdominal Stent Graft System. The ALTO system is particularly suited for patients with short aortic necks and has helped the company strengthen its presence in the anatomically challenging segment. Endologix currently commands roughly 8% of the global market.
Terumo Corporation and Becton, Dickinson and Company are emerging players in the market, collectively holding around 10–12% of the share. Terumo’s acquisition of Bolton Medical has enhanced its position through the Relay and Treovance product lines. Bard Peripheral Vascular, now part of BD, markets the Flair Endovascular Stent Graft and continues to penetrate the hospital procurement ecosystem through its extensive vascular access product lines.
Product Line Strength and Strategic Positioning
Medtronic’s Endurant series is widely recognized for its applicability in both straightforward and complex AAA cases, and its global clinical trial data offers physicians strong confidence in patient outcomes. The company continues to expand its clinical indications, enhancing adoption in both inpatient and outpatient settings.
Cook Medical stands out for its robust physician support model. The Zenith Fenestrated platform allows tailored fenestration for renal and visceral arteries, enabling successful treatment in anatomies previously considered unsuitable for EVAR. Its commitment to patient-specific design positions it well in advanced care centers.
L. Gore has focused on simplifying procedures while maintaining high clinical success rates. The Excluder Conformable AAA Endoprosthesis, introduced as a successor to earlier Excluder models, offers enhanced anatomical conformability. This feature addresses one of the most common causes of post-procedural complications—poor device fit and migration.
Endologix, despite operational restructuring, remains competitive through its innovation pipeline. The ALTO system’s polymer-based sealing mechanism has been praised for its adaptability in short aortic necks, giving it an advantage in high-risk populations. Additionally, the company’s continued focus on complex cases allows it to serve a specialized segment of the abdominal aortic aneurysm repair device market.
Recent Developments and Industry News in the Abdominal Aortic Aneurysm Repair Device Market
The abdominal aortic aneurysm repair device market has seen a number of important developments in recent years that reflect ongoing innovation and strategic realignments.
In July 2022, a leading global manufacturer announced the completion of a major clinical trial for a next-generation fenestrated stent graft system, focusing on long-term durability in complex abdominal anatomies. The results are expected to support an expanded regulatory approval pathway across both the United States and Europe.
In March 2023, a new modular endograft system featuring self-expanding technology was launched in Asia-Pacific. This product targets low-profile deployment for patients with tortuous iliac arteries and has been integrated into outpatient treatment programs in urban centers.
September 2023 marked a strategic collaboration between a top-tier stent graft manufacturer and a major imaging company to co-develop real-time procedural guidance software. This software integrates with stent graft deployment systems, offering intraoperative feedback on device position, wall contact, and sealing zone optimization.
In January 2024, Endologix announced the commercial expansion of the ALTO system into Eastern European markets, with distribution agreements finalized in Poland, Hungary, and the Czech Republic. This move aligns with the company’s goal to increase its global footprint in underpenetrated markets.
More recently, in April 2024, a U.S.-based medical technology firm entered the abdominal aortic aneurysm repair device market with a customizable graft system designed for outpatient use. This development reflects the ongoing shift toward same-day discharge procedures and may reshape product design and procurement priorities over the next two years.
“Abdominal Aortic Aneurysm Repair Device Production Data and Abdominal Aortic Aneurysm Repair Device Production Trend”
-
-
- Abdominal Aortic Aneurysm Repair Device sales database for historical years, 10 years historical data
- Abdominal Aortic Aneurysm Repair Device sales data and forecast for next 7 years
-
Key Insights that the Abdominal Aortic Aneurysm Repair Devices Market analysis report presents are:
- Abdominal Aortic Aneurysm Repair Devices Market revenue and demand by countries
- Break-down of the Abdominal Aortic Aneurysm Repair Devices Market in terms of application areas, target customers, and other potential market segments
- Areas that are relatively more potential and are faster growing
- Abdominal Aortic Aneurysm Repair Devices Market competitive scenario, market share analysis
- Abdominal Aortic Aneurysm Repair Devices Market business opportunity analysis
Global and Country-Wise Abdominal Aortic Aneurysm Repair Devices Market Statistics
- Global and Country-Wise Abdominal Aortic Aneurysm Repair Devices Market Size ($Million) and Forecast – (till 2030)
- Global and Country-Wise Abdominal Aortic Aneurysm Repair Devices Market Trend Analysis
- Global and Country-Wise Abdominal Aortic Aneurysm Repair Devices Market Business Opportunity Assessment
“Every Organization is different and so are their requirements”- Datavagyanik
Companies We Work With






Do You Want To Boost Your Business?
drop us a line and keep in touch

